A post-market review of disposable face masks and respirator products revealed one in four samples failed to properly filter out small, aerosolised particles.
The TGA has tightened the criteria for face masks and other respirator products to be entered on the ARTG after a post-market review of items added to the register during the early stages of the covid pandemic found many were non-compliant with various safety and quality standards.
The covid pandemic saw the number of disposable face masks and respirator products included in the Australian Register of Therapeutic Goods skyrocket from 102 prior to February 2020 to 1183 in May 2020 due to increased demand for said products.
The TGA subsequently received a significant amount of feedback from healthcare workers, the public and government officials regarding the quality and safety of these products.
The feedback led to the then Department of Health and Aged Care to undertake a post-market review of the face masks, and the findings of the report were published earlier this month.
“The objective of the review was to verify whether these products met regulatory requirements and performed as intended, and in turn, inform the public of the safety and effectiveness of face masks available for supply,” the report said.
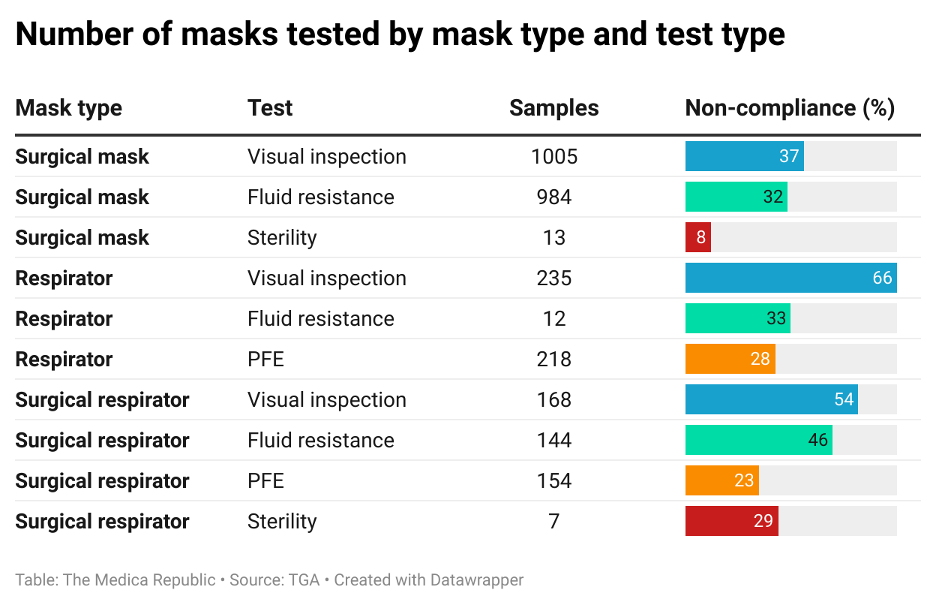
As part of the review, 1568 samples of 957 surgical masks, respirators and surgical respirators registered on the ARTG were subjected to a series of tests, namely:
- A visual and physical inspection of the labelling design and construction quality of the product to determine the device met the requirements established by the manufacturer, were consistent and defect-free and complied with labelling and packaging requirements.
- Fluid resistance testing to determine if and how much fluid can penetrate the face mask materials.
- Particle filtration efficiency (PFE) testing to assess the products’ ability to filter out aerosolised particles smaller than 300nm, and whether the filtration efficiency value met the advertised classification.
- Sterility testing to identify any contamination that may have occurred during the production, sterilisation or shipping process.
All tests were performed according to the relevant standards, procedures and/or methodologies.
More than 20% of samples tested were non-compliant across each of the four tests, with the only exception being the 8% of surgical mask samples that were non-compliant with sterility requirements. Respirators – surgical or otherwise – displayed greater levels of non-compliance than surgical masks.
Related
When considering all products, 44% were non-compliant on visual inspection, 34% were non-compliant with fluid resistance requirements, 26% were non-compliant with PFE testing, and 15% were non-compliant with sterility.
Although the post-market review included 2276 ARTG entries up until the end of May 2023, two thirds of these entries were cancelled from the ARTG by the sponsor and a further 23% were cancelled by the TGA.
In addition to these cancellations, the TGA also imposed conditions of inclusion for devices being listed on the ARTG, sought variation to Global Medical Device Nomenclature codes (an international naming and grouping convention used to identify and consistently describe medical devices) and issued product defect alerts and recalls.
“The post-market review and testing of face mask products, along with subsequent regulatory actions, were effective in identifying and removing a significant number of non-compliant products from the ARTG. This helped to ensure that the remaining products available for supply in Australia are suitable for their intended purposes,” the report concluded.
“Changes to the ARTG inclusion process for class I medical devices also helped reduce the risk of non-compliant products being supplied in the future.”